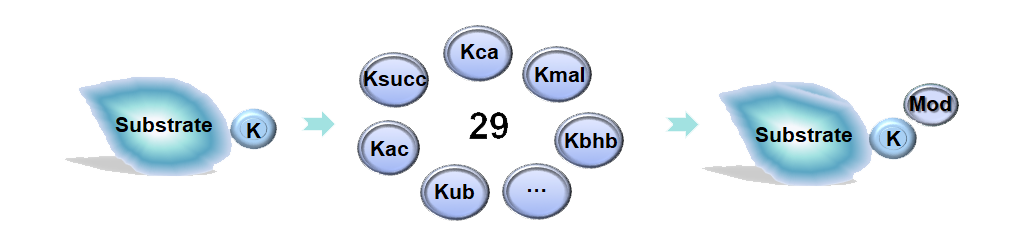
Protein lysine modifications (PLMs), a subgroup of protein post-translational modifications (PTMs), specially occur at lysine residues in proteins, and play important roles in regulating a broad spectrum of biological processes and pathways. With the rapid progress of mass spectrometry-based technology and development of PLM-specific antibodies, many novel PLM types as well as substrates and sites have been identified to be associated with human physiology and disease. For example, a recently discovered PLM, β-hydroxybutyrylation, could promote the malignancy of hepatocellular carcinoma (HCC) (Zhang, et al., 2021).
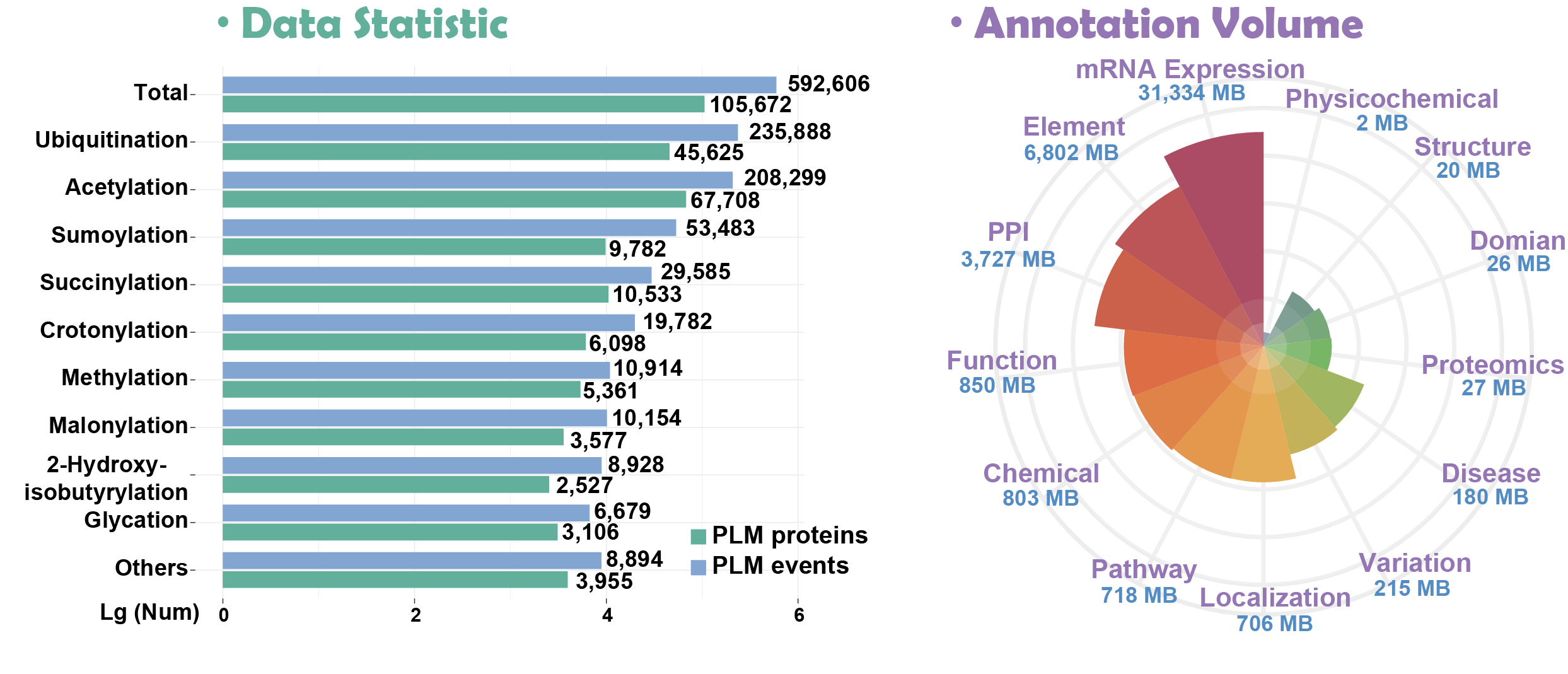
Here, we present Compendium of Protein Lysine Modifications 4.0 (CPLM 4.0), a well-annotated data resource extended and updated from previous versions including CPLA 1.0 (Liu, et al., 2011), CPLM 2.0 (Liu, et al., 2014) and PLMD 3.0 (Xu, et al., 2017). Currently, CPLM 4.0 contained 592,606 modification events in 463,156 unique sites of 105,673 proteins for up to 29 PLM types across 219 species.
more details >>> hide details <<<In addition, we provided detailed annotations for these proteins using the knowledge of 102 public databases that covered 13 aspects: (i) Variation and mutation; (ii) Disease-associated information; (iii) Protein-protein interaction; (iv) Protein function; (v) DNA & RNA element; (vi) Protein structure; (vii) Chemical-target relation; (viii) mRNA expression; (ix) Protein expression/Proteomics; (x) Subcellular localization; (xi) Biological pathway; (xii) Domain annotation; (xiii) Physicochemical property. All datasets and annotations are free for use.
※ Statistics
 |
|
For publication of results please cite the following articles:  CPLM 4.0: an updated database with rich annotations for protein lysine modifications
CPLM 4.0: an updated database with rich annotations for protein lysine modifications Weizhi Zhang, Xiaodan Tan, Shaofeng Lin, Yujie Gou, Cheng Han, Chi Zhang, Wanshan Ning, ChenWei Wang and Yu Xue. Nucleic Acids Research. 2021, 44 (5): 243–250. [Abstract] [FREE Full Text] [PDF][Supplementary Data]  PLMD: an updated data resource of protein lysine modifications
PLMD: an updated data resource of protein lysine modifications Haodong Xu, Jiaqi Zhou, Shaofeng Lin, Wankun Deng, Ying Zhang, and Yu Xue. Journal of Genetics and Genomics. 2017, 44 (5): 243–250. [Abstract] [FREE Full Text] [PDF][Supplementary Data]  CPLM: a database of protein lysine modifications
CPLM: a database of protein lysine modifications Zexian Liu, Yongbo Wang, Tianshun Gao, Zhicheng Pan, Han Cheng, Qing Yang, Zhongyi Cheng, Anyuan Guo, Jian Ren, and Yu Xue. Nucleic Acids Research. 2014, 42: D531-D536. [Abstract] [FREE Full Text] [PDF] [Supplementary Data]  CPLA 1.0: an integrated database of protein lysine acetylation
CPLA 1.0: an integrated database of protein lysine acetylation Zexian Liu, Jun Cao, Xinjiao Gao, Yanhong Zhou, Longping Wen, Xiangjiao Yang, Xuebiao Yao, and Jian Ren, Yu Xue. Nucleic Acids Research. 2011, 39: D1029-D1034. [Abstract] [FREE Full Text] [PDF] [Supplementary Data] |
 |